Pharma Part 4: Cipher Pulls the Trigger $CPH.TO
Doubling Profits and Returning to Growth with the Acquisition of Natroba
TL;DR
Cipher Pharma’s acquisition of ParaPRO’s Natroba assets and operations have transformed the company from a conservatively financed melting ice cube with a call option on a promising but speculative toenail fungus treatment, to a moderately leveraged growth company with many avenues to deploy capital profitably, of which the toenail fungus treatment is just one.
The stock has risen ~30% since the announcement (from ~$9 CAD to ~$12); however, CPH’s pro forma EV/EBITDA multiple has hardly changed, while the business clearly has.
I think the stock is considerably more attractive at $12 and the new assets, than it was at $9 without them.
I personally owned it at $9 and added at $12.
There is a bullet point summary at the end if readers want more details but don’t want to read the whole thing.
Introduction:
This is Part Four of the toenail fungus saga; my original write-up covering Cipher and toenail fungus is here; Part Two, covering the Swedish developer of the toenail fungus drug, Moberg Pharma, is here; and most recently, how I became convinced the hype is real is in Part Three here.
As usual, I will remind readers that I am very much an amateur who has just read a lot of pubmed and public filings in the last few days, so don’t take this as advice. I also owe a lot of the thinking here to others, like Left’s substsack and Divergent Capital’s write-up, in addition to everyone on twitter who’ve been brainstorming.
We pick up the story now, with Cipher deploying its cash pile to buy from ParaPRO the operations (mostly a USA-based ~50-person sales team) and the rights to the drug Natroba, used to treat lice and scabies.
Press release is here.
Terms of the deal
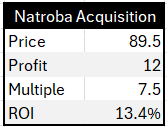
Cipher paid USD $40M in cash, $40M in debt, and $9.5M in stock, for a total of $89.5M. Some will wonder about the stock issuance. Why not just more debt? Here is the answer:
Having the leaders of the acquired company stay on and take significant amounts of stock in the deal is a good sign, in my opinion. I think it implies the sellers see upside in the deal, want to participate, and will have skin in the game to create good incentives.
In terms of numbers, Mull said in the press release the acquisition will double earnings (by which he meant EBITDA). Because Cipher makes around $12M in EBITDA, we can infer Natroba does too. The implied EV/EBITDA multiple paid by Cipher is ~7.5x (89.5 / 12). Assuming no growth or synergies, the return on investment is ~13-14% (12 / 89.5).
What are they getting for that price? Let’s find out.
Asset #1 – Natroba for Lice
Natroba is the brand name for the drug Spinosad, an effective anti-parasitic used to treat head lice and scabies.
For lice, Natroba is at an immediate disadvantage because it requires a prescription while other treatments, like the first-line treatment 1% permethrin, do not. It is also off patent for treatment of lice, meaning generics may begin to emerge. That said, compared to other options, Natroba is highly effective, well-tolerated, and convenient. Let’s discuss:
Firstly, Natroba kills lice at about double the rate of 1% permethrin, the current first-line treatment and standard of care. Here are the numbers:

Secondly, it is convenient compared to other treatments. Unlike permethrin (and some other treatments), spinosad kills lice eggs not just the lice (the term is “ovicidal”), meaning a single treatment (and no tedious combing) is usually all that’s required to de-louse a person’s head. Non-ovicidal treatments typically require at least two since they only kill the lice and not the nits/eggs.
In addition to not requiring combing of nits (the eggs), a patient puts it in their hair for 10 minutes then washes it out. This compares favourably to a drug like malathion, which requires it be left in the hair for 8-12 hours. Spinosad is relatively convenient for users.
Lastly, unlike some other ovicidal treatments like Ivermectin or Lindane, and even the tranquil 1% permethrin, Spinosad is relatively well-tolerated, with few people experiencing side effects or reactions.

Here’s a decent summary from GoodRx:
This is all in the context of increased resistance to traditional treatments. Here, for example, is part of a discussion on that topic from 2014:

1% permethrin in 2014 was down to as low as 30% efficacy. The article goes on later to say that newer treatments are efficacious but are often more costly and require a prescription.
As efficacious as Natroba may be, being off patent, expensive, and unavailable over the counter in the USA probably limits growth in that geography. Natroba could get approved in other jurisdictions however, like in Cipher’s backyard of Canada for example.
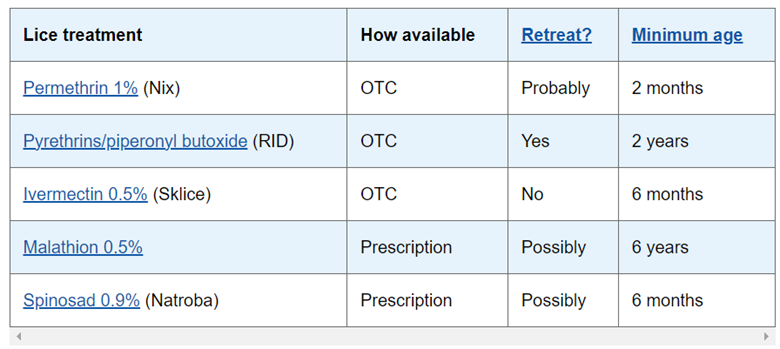
Because Canada is a small market in dollar terms (and not worth the time for many large conglomerates, eg Bayer’s “RID” lice treatment), patients have relatively few options other than the incumbent permethrin/pyrethrin. These are them:

They all claim high effectiveness, but none are insecticides, while Spinosad is, suggesting Spinosad likely has an edge. Moreover, none are ovicidal (meaning multiple treatments will be required). Additionally, Natroba uses the third option, benzyl alcohol, in its Spinosad solution, making that medication redundant. In sum, Canada’s market for lice treatment is looking ripe for disruption by a product like Natroba, and there are probably many other countries like it.
Overall, while Natroba for lice may be more convenient, more effective, and less toxic than competitors, it is not available over the counter and has gone off patent in the USA, which probably limits its economic potential there. That said, incremental growth for Cipher is possible by getting it approved in other countries, like Canada.
Asset #2 – Natroba for Scabies
Natroba’s 2021 indication for scabies is what Cipher shareholders should be more excited about, in my opinion.
This part of the discussion ended up being long and a little tedious, so if you just want some facts and to skip to the next heading, here’s a summary:
Natroba is FDA-approved for scabies, has a high cure rate (even by the more rigorous modern standards), conveniently requires just one dose, and is well-tolerated, with very few reactions and/or adverse effects.
Natroba for scabies is patent protected until 2033, so competitors won’t be able to copy them.
Natroba has no over the counter competition, unlike the lice indication.
The market’s leading standard of care, 5% permethrin, has rapidly deteriorating efficacy, and alternatives are going to be required.
None of the alternatives are perfect, Natroba’s drawback is that it has only two proper studies, but that is a better drawback than many competitors.
200-400 million people have scabies, meaning there is room to grow outside of the USA, though relatively few of those will likely be able to pay too much.
Overall, Natroba for scabies is a defensive growth asset in a niche market.
And now the full discussion…
What lice is to your scalp, scabies is to your skin. It is extremely common globally, with 200-400 million people having the condition. Many of these are in developing countries (where drug prices are generally lower), but even the USA has around one million cases at any given time, according to the press release. We’ll focus on the US for now, since that is the only jurisdiction where the drug is currently approved.
Significantly, there are no over the counter competitors for scabies and Natroba has patent protection until 2033 on Spinosad’s scabies indication, so Natroba has a much greater chance of wider adoption with less chance of competition.
In this market, 5% permethrin (a stronger version of the permethrin for lice) is the market leader. According to the Center for Disease Control, along with Ivermectin tablets for more severe cases, these two medications represent first-line treatment:

The cure rate for 5% permethrin is a little tough to pin down because the definition of cured changed in 2016, going from a scientist simply observing the change to tests being done to verify the researcher’s observations; the FDA calls this new, more rigorous standard, “complete cure.”

For example, these two studies in 2012 and 2013 had courses of 5% permethrin and/or Ivermectin at well above a 90% cure rate, presumably using the old definition of cured. Whereas in a more recent study comparing a compound available in Europe (benzyl benzoate) to 5% permethrin, in which they objectively tested the patients for scabies eradication (“dermoscopy-verified”) rather than just observing them, 5% permethrin came out with a much worse cure rate, only 27%:

A drop of around 60% efficacy is quite dramatic. That is just one study (and probably paid for by the benzyl benzoate folks), but there are more. This one from 2022, comparing sulfur lotion to 5% permethrin warned, “The resistance to 5% permethrin cream … can be a concern as an unexpected low success rate was obtained in the study.”
Lastly, this one published in 2022, again verifying objectively in addition to subjectively, corroborated the findings of the more recent studies, that 5% permethrin’s cure rate is around 30%:
The authors of this last study believe that the loss in efficacy is due to increasing resistance of the scabies mite to permethrin, but I wonder to what extent the methods for determining cure played a part as well.
Somewhat rebelliously, the authors conclude that because official guidelines don’t factor in the reduced efficacy of 5% permethrin, “clinical applicability is limited.” In other words, the medical community may want to look at alternatives to the standard of care. Here is what the CDC suggests:
It’s clear why they are classified as alternatives; they all have some sort of issue. Crotamiton isn’t very effective, sulfur stinks, malathion has little research, Lindane is quite toxic, and Spinosad, the one Cipher now owns, has only two studies with small sample sizes. Here’s the the summary of those two studies:
Complete cure rate is 70% and 84%, which sounds pretty good, especially considering participants only took one dose, rather than the two most treatments require (like 5% permethrin and oral ivermectin which take a second dose a week later to kill the hatched eggs).
However, readers may have noticed that Natroba’s vehicle for Spinosad is doing a lot of work as well; just the vehicle (without the Spinosad in it) cleared almost half the scabies in the first trial and 35% in the second. That is ordinarily negative (since vehicles typically are not medicinal), but Natroba’s vehicle contains benzyl alcohol (which you may recognize as an option to treat lice in Canada) and is related to a European first-line treatment for scabies, benzyl benzoate, that was favourably compared to 5% permethrin in a study discussed earlier (87% cure rate).
In short, Natroba seems to have two active ingredients, one an insecticide and one not. Is this a bad thing? I’m not an expert so I don’t really know, so would love feedback on this point.
That said, I can see why Natroba is on the alternative list. There are only two studies, both with relatively small sample sizes, in which the vehicle for the drug did almost half the work.
On the other hand, very few drugs for scabies have two proper clinical phase three trials since the FDA began requiring objective verification in addition to investigator’s observations. Recent studies under the more rigorous definition of “cured” involving 5% permethrin, the current standard of care, gave the drug such poor marks that it makes me wonder whether it would even be approved today.
Natroba brags a little about being the first FDA approved indication for scabies in 30 years and that it’s been done under the new more rigorous complete cure criteria – and I’m beginning to see why. Whether prescribers and health agencies see though, we’ll have to wait to find out.
I’m curious to browse the financials because I want to know growth rates and sales for Natroba’s lice and scabies indications. They claim to have 22.1% of the anti-parasitic market, which seems like quite a lot for a prescription medication, so I eagerly await the numbers.
Nonetheless, at ~7x EV/EBITDA and already plenty of ways to grow Natroba, Cipher is looking like it bought Natroba with a reasonable margin of safety. The value guy in me loves that.
Asset #3 – US-Based Salesforce
From the press release:
This is significant because Cipher does not currently have a salesforce in the USA and is forced to out-license its products there, collecting a royalty rather than all the profits. This brings several opportunities.
Firstly, and most immediately, I see Cipher bringing their main out-licensed product, Absorica (an isotretinoin for severe acne), in-house. The current license expires in 2026 and is held by Sun Pharma. Sun Pharma recently renegotiated Cipher’s royalty down and is marketing competing acne drugs, meaning Cipher may be extra motivated to do its own US sales.
Twitter recently napkin mathed what would happen if Absorica were brought in house. I’ve summarized it here:
Keeping in mind this is speculation, Cipher currently makes $6M USD in EBITDA on Absorica in the USA and could make $18M if they bring it in-house, for a $12M gain, doubling current EBITDA and bring the ROI of the acquisition from ~13% to ~25%. The synergy is phenomenal; even if Sun Pharma were a perfect partner, this must be attractive to management.
Secondly, Moberg Pharma will be auctioning the license to sell MOB-015 to dermatologists in the USA following the January read-out of the final phase three trial. Now that Cipher owns a dermatology platform in the US, they will be able to bid for it. It’s a long shot, as there will be a lot of well-heeled pharma companies bidding for a drug that could do huge sales, but just the possibility increases the right tail of probabilities strongly.
Lastly, Cipher has far more options for in-licensing and acquiring than before. The company will be making more money and have operations in two markets, one of which is the biggest in the world. CEO Craig Mull has impressed so far with his capital allocation and this new platform and cash flow gives him more opportunity to do well for shareholders.
To sum, the 50 person US-based salesforce could double EBITDA if they bring Absorica in-house, allows them to bid for the US license to MOB-15, and opens a lot more investment opportunities.
Relevant Developments
Moberg released MOB-015, under the name “Terclara,” in Sweden. Part of the thesis was that it would take market share from incumbents and had the potential to expand the market itself. Both are panning out:
After only one month of consumer advertising Terclara is the market leader for toenail fungus treatments and grown the total market for toenail fungus treatments by 52%. This is obviously bullish for Cipher, which owns the rights to the drug in Canada.
The main difference between Canada and Sweden is that Terclara is available over the counter whereas in Canada it will be prescribed. This would slow adoption of MOB-015; becoming the market leader in one month is probably not possible.
Canada is so short doctors however, that pharmacists are being given expanded roles, including prescribing for minor ailments. In 2023 for example, the province of British Columbia made it legal for a pharmacist to prescribe topical drugs for fungal infections, including onychomycosis (toenail fungus):

In BC, Canada, MOB-015 will technically be a prescription medication, but can effectively be sold over the counter. And this is happening all over Canada, including in the most populated provinces of Ontario and Quebec, though their lists of minor ailments don’t currently include onychomycosis. Itching and insect bites are included though, which are not too far off from lice and scabies.
Overall, the momentum is great for Cipher. MOB-015 is doing what we thought it would and Canada is making it easier to get prescription medications. We’re still far from certain how exactly this will play out, and I’ll be following both developments closely, but I am more optimistic than before.
Financial Model and Valuation
I’m using mostly the assumptions from the SIB Cipher submitted, that modelled the contributions of MOB-015 in the future, and Mull’s statement in the press release saying Natroba will double profits (EBITDA) for the company. The rest is my own estimation/speculation.
Here it is:
We’re going to disagree on the assumptions here, especially on when and to what degree MOB-015 rolls out in Canada. However, a lot has been left out, likely making up for at least some of that downside uncertainty with upside uncertainty.
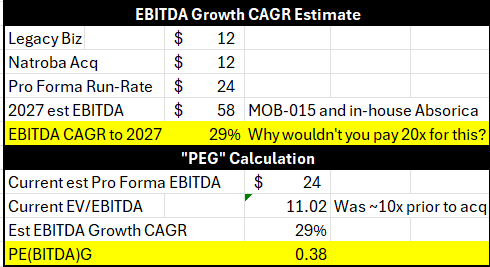
What do you pay for a company that could do close to $60M in EBITDA in 2027, and is only doing $24M (pro forma) now? I did a little “PEG” calculation to get a rough idea what the market thinks:
Readers can make up their own minds and models, but the fact is the stock is trading at 11x EV/EBITDA when it was trading at 10x prior to the acquisition of ParaPRO, while the company has changed significantly, in my opinion.
Summary:
Cipher bought ParaPRO’s Natroba…
for $89.5M USD
a multiple of around 7.5x.
the former CEO of ParaPRO took almost $10M in shares as part of the deal.
Natroba for lice…
is effective, convenient, and non-toxic.
is prescription while some competitors are available over the counter.
is not patent protected.
could be grown incrementally by expanding to other markets.
Natroba for scabies…
is patent protected in the USA until 2033.
was FDA-approved in 2021 under new (more rigorous) testing.
has tested clinically to have a high cure rate of ~78%
Around 40% of that is the vehicle.
Limited by having only two proper studies
has no over the counter competition.
The standard of care, 5% permethrin, has lost much of its efficacy in the last ten years (~70-90% cure rate in 2012 to ~30% today).
Safe, effective, and approved alternatives like Natroba will be required
200-400 million people in the world have scabies, mostly in developing countries (who probably can’t pay much).
Growth will likely come organically in USA and by expanding to other geographies.
Natroba’s salesforce…
Allows Cipher to bring Absorica in-house, potentially increasing profits from a $6M royalty to $18M in operating income.
Allows Cipher to bid for MOB-015’s US derm license.
Opens up reinvestment opportunities for management.
Updates
MOB-015 or “Terclara” is the market leader in Sweden and expanded the market itself.
Canada is letting pharmacists prescribe for certain minor ailments, making some prescriptions more like over the counter drugs, and therefore far more accessible.
Model and Valuation
11x EV/EBITDA (at ~$12/share) vs 10x before the acquisition was announced.
I estimate Cipher could grow EBITDA at a 30% CAGR to 2027, before incremental growth of Natroba and others.
Conclusion:
There is obviously a lot we don’t know, but the company has likely transformed from a conservatively financed melting ice cube with a call option on a promising but speculative toenail fungus treatment, to a moderately leveraged growth company with many avenues to deploy capital profitably, of which the toenail fungus treatment is just one.
Natroba appears a quality asset, but I doubt it compares to MOB-015, mostly because more wealthy people have toenail fungus than scabies, and competition is much less. MOB-015 is unique though, and with this acquisition CEO Craig Mull has continued to impress.
We’ll know more with earnings and as management shares more details, but the direction is good. Multiple expansion seems likely, and forward returns look strongly positive, in my opinion.
I owned it at $9 a share and added at $12.
Not advice, just opinion. Remember that I’m just a school teacher, not an expert.














I wanted to comment on Natroba using Benzyl Alcohol as a vehicle. It seems there is a number of different reasons why it could be included. This is from Claude.
Preservative:
It has antimicrobial properties, helping to prevent bacterial and fungal growth in the cream.
This extends the shelf life of the product.
Solvent:
It can dissolve certain ingredients that might not be soluble in water or oil alone.
This helps in creating a stable and homogeneous formulation.
Local anesthetic:
Benzyl alcohol has mild anesthetic properties, providing a slight numbing effect.
This can help reduce itching or minor skin irritations.
Penetration enhancer:
It can slightly enhance the penetration of other active ingredients through the skin.
Viscosity modifier:
It can help adjust the thickness and texture of the cream.
Emollient:
In some formulations, it may contribute to the moisturizing properties of the cream.
FDA approval:
It's generally recognized as safe (GRAS) by the FDA for topical use in limited concentrations.
Very well done