Moberg & Cipher – The Final Puzzle Pieces - $MOB.ST $CPH.TO
Data to draw the picture, Stories to colour it in, and an Analogy for perspective
Introduction:
This is the third article of what’s become a fiur-part series on investment opportunities in a novel toenail fungus treatment, MOB-015 (branded as Terclara).
I’ll be assuming you’ve read the first article, which covered Cipher Pharmaceuticals, the company that has the rights to MOB-015 in Canada, the second, which covered Moberg Pharma, the creator of MOB-015, and here is the fourth, covering Cipher’s acquisition of a new product, which you can read after this one.
I didn’t have enough conviction in the ability of MOB-015 to expand the market for treatment or in the probability of positive results coming from the ongoing phase three trial to buy either stock in any size.
After reading Moberg’s patent extension application and exploring the subreddit “r/toenailfungus,” I have a much clearer picture and will certainly be adding to both Moberg and Cipher over the course of this year.
Puzzle Piece #1 – The Data
The patent application really lays out the thesis perfectly, just in plenty of lawyer and scientist jargon. I’m no lawyer or scientist (I’m a teacher in fact), so please read the document yourself – my analysis here is just a supplement.
Firstly, the reason topical treatments up to now are not very effective is because the toenail is a hard thing to penetrate:
To penetrate the nail, the nail must be hydrated, and no one had figured out how to do it well with a topical formulation - until Moberg. MOB-015 is effective because it manages to hydrate the nail enough to deliver high concentrations of the antifungal (terbinafine) to the nail bed (where the fungus is).
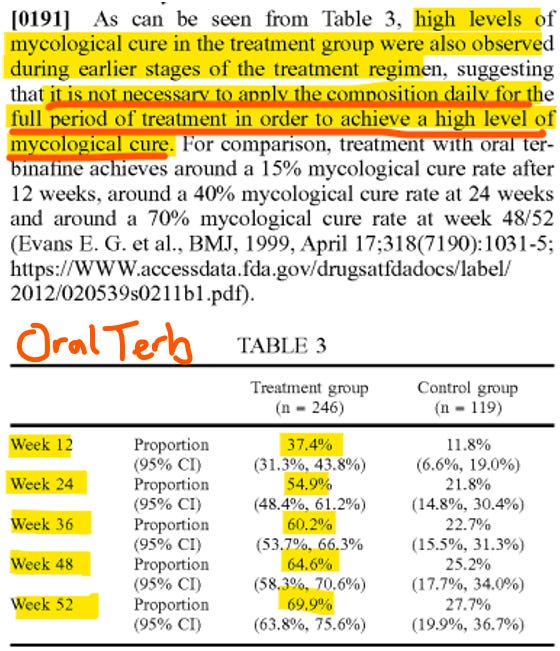
Here’s the data on MOB-015 and competition:
From a mycological cure perspective, MOB-015 matches and even bests oral terbinafine and clearly outpaces the topical competition. However, as the highlighted note at the bottom suggests, the complete cure rate (returning the nail to normal appearance), is confusingly low.
Normally if you cure the fungus, the nail follows. Not so with MOB-015. The low complete cure rate is almost certainly a big reason a company disrupting a $3-5B market can trade at less than USD $100M market cap. How could the mycological cure be so high and the complete cure so low? Something doesn’t add up.
If you investigate the numbers, the reason complete cure is so low relative to mycological cure is because the product works too well – MOB-015 overhydrates the nail, resulting in a permeable nail but also causes a whitening effect on the nail that can’t be said to have been returned to normal appearance (“completely” cured):
According to the patent extension application, they have an idea and even some evidence as to how to fix this (relatively good) problem. When patients stopped treatment for a month the nails appeared to “dry out,” meaning adjusting the dosage could improve complete cure rates.
This is what the current (and third) phase three trial is testing – a change in treatment regimen/dosage to maximize complete cure without reducing mycological cure:
The “loading” phase is 8-12 weeks of daily application (rather than for a full 48 weeks as with other topicals), followed by a “maintenance” phase of approximately weekly application to allow the nail to dry out, reducing the chances of overhydrated/white nails at the end of treatment.
Readers might be asking whether this will risk lowering the mycological cure rate. We know this reduced dosage is very unlikely to lower mycological cure because a complete course of oral terbinafine (the same active ingredient as is in MOB-015) lasts 8-12 weeks but has effects for a whole year. Here’s a chart provided in the patent application:
If terbinafine in sufficient concentrations is in the nail bed for 12 weeks, 36 weeks later the fungus is usually gone – like oral terbinafine, MOB-015 achieves this, so Moberg has some leeway to optimize for complete cure later in the treatment. It even works quickly, with visible results by 12 weeks, which is sure to help patients complete their treatment.
So, what’s left to do is mostly just tweak what’s already an effective treatment. To me, this means that the probability that the ongoing phase three trial is successful is much higher than I expected, and the drug is much higher quality than I supposed. Complete and mycological cure rates matching or exceeding oral terbinafine (but without the dangers) are the most likely outcome. MOB-015 is a lot less speculative than the financials and clinical data appear to suggest.
The patent summarizes it best:
Put simply, existing treatments are effective but unsafe or safe but ineffective – MOB-015 is the only nail fungus treatment to be both safe and effective in the world. This isn’t news but reading the patent extension application really helped take this fact from “knowledge” to “understanding” in my head.
The question investors ask next is what type of effect will that have on patients and the market for treatment? To answer this question, I initially looked at data but found it didn’t really enhance my depth of knowledge – I couldn’t really understand how real people would react to MOB-015 just from data.
Puzzle Piece #2 – The Stories
Subreddit r/toenailfungus on the other hand is loaded with stories that take us right into the mind of MOB-015’s target customers. I’m going to share a few screenshots that I found extremely helpful for estimating what impact MOB-015 might have on real people.
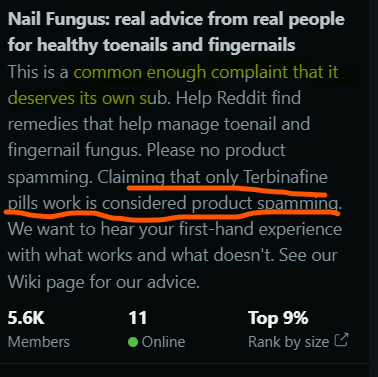
Firstly, here’s the subreddit’s description and information:
“Nail fungus is a common enough complaint that it deserves its own sub,” is a promising start for Terclara sales. It also seems to be true as it ranks in the top 9% of subreddits in terms of size.
Also of interest is that the moderator seems to want to limit people claiming only terbinafine pills work, implying enough people feel this way that we really must question the efficacy of the other products (as we have done).
The moderator continues in this vein:
Doubling down on the terbinafine-pilled, the moderator has to reiterate that, “We can’t all take the pills even though it is considered the gold standard.” In other words, the gold standard, terbinafine pills, are dangerous enough plenty of people don’t or can’t use them. You’d think it doesn’t get more bullish for an effective topical treatment than this, but it does.
This person spent twenty years trying to get rid of her nail fungus and eventually had to grind her nails down and take terbinafine pills (which required blood tests to make sure her liver wasn’t getting poisoned) - “I could literally cry I’m so happy.” The market for an effective and safe treatment is there.
Look at this one for example:
My main takeaway from this one is that this person had the fungus and left it untreated for ages because they were so worried about the safety of the pills. They write, “Just take the medication.” To me, this suggests there are plenty of people with toenail fungus out there trying to fix it that aren’t prescribed to anything – the white space is large.
Take this guy for example:
People are basically trying to hocus pocus the fungus away rather than take the existing medications. Vicks? Cream of tartar? Bleach? Blaming diet?
Even more extreme, this person literally had their toenails pulled out:
In other posts I saw just about everything from homeopathic stuff to tea tree oil to actual fungicide:
Here’s a conversation in the comments of one of these posts:
“It takes forever to see results,” “I stopped [terbinafine pills] because it can damage the liver,” and “… try using a heavy-duty nail file or even a Dremel” stand out most to me. These people are desperate.
The point of these anecdotes is to “colour in” the data, and show that the existing treatment options are not even coming close to meeting the need. So investors must ask themselves, “How would these people react when they hear about a quick-acting, safe, and effective topical treatment?”
I’ll let readers use their own judgement to answer that question, but I feel toenail fungus sufferers might be willing to give Terclara a go - sounds a lot better than taking a Dremel to your toenails anyways.
Conclusion - An Analogy
Flaky dandruff is a fungal condition that affects around the same number of people as toenail fungus. Until 15-20 years ago, it was treated predominantly with an oral antifungal (ketoconazole) that caused liver damage in 1:15,000 to 1:20,000 people, and had numerous potential side effects and dangerous drug interactions. That medication has largely been supplanted (and even banned in some jurisdictions) by topical formulations from brands like Nizoral.

To gain perspective, it might be helpful to think of toenail fungus in a similar place to dandruff 15-20 years ago. The main difference is that the leading oral treatment (also with liver toxicity, side effects, and drug interactions, just not as severe) is still considered the “gold standard” because topicals must penetrate a nail to get to the skin, which they currently can’t do – Moberg’s Terclara can.
There are plenty of details to iron out, like pricing, marketing, awareness, availability, funding, etc but I think if we zoom out, we can say the path of least resistance is for toenail fungus to go the way of dandruff: from a stubborn, dangerous to treat, and mildly stigmatized condition, to an easy, safely-treated, and commonly understood ailment. Moberg and its licensees, like Cipher, are the cause and beneficiaries of this emergent reality.
As you can tell from the fact I’ve lost some of the dispassion with which I usually write, I’ve officially booked my ticket on the Moberg/Cipher train, and it’s only just leaving the station - in my opinion.
Thanks for reading!


















Really good detail on the effectiveness of MOB-015. Welcome to the club.
New PR as of today regarding the sales in Sweden since launch in February
Moberg Pharma AB's (OMX: MOB) reports strong interest from pharmacists, doctors and end customers in the launch of MOB-015 under the Terclara[®] brand in Sweden. A majority of Swedish pharmacies now have the product available on the shelf.
Interest in the product exceeds the chains' forecasts, and the pharmacy chains are increasing their orders due to the fact that Terclara® has occasionally sold out at several of the pharmacy chains. However, there is a well-stocked wholesale warehouse.
https://www.mobergpharma.se/pressmeddelanden/2024-04-23/lanseringsuppdatering-mob-015